Organ Bioengineering
Organ Bioengineering: Creating Bioengineered Organs and Liver Constructs for Regeneration, Transplantation, and Advanced Disease Modeling
Organ bioengineering represents a transformative approach in modern medicine, aiming to overcome the limitations of donor organ shortages and improve therapeutic outcomes for patients with end-stage organ failure. This discipline integrates principles of tissue engineering, regenerative medicine, and cellular therapy to create functional, bioengineered organs that mimic the structural and physiological properties of native tissues. Among these, liver constructs have emerged as a critical focus due to the liver’s central role in metabolism, detoxification, and homeostasis.
The process begins with decellularization, where donor organs—human or xenogenic are stripped of cellular components while preserving the extracellular matrix (ECM) architecture. This ECM serves as a natural scaffold, maintaining vascular networks and biochemical cues essential for cell adhesion and growth. Subsequent repopulation with stem cells or hepatic progenitors enables the restoration of organ functionality. Advanced bioreactor systems provide controlled environments for nutrient delivery, oxygenation, and waste removal, ensuring optimal cell viability and differentiation.
Bioengineered liver constructs are not only pivotal for transplantation but also serve as disease modeling platforms. These constructs allow researchers and clinicians to simulate pathological conditions such as fibrosis, cirrhosis, and viral hepatitis in a three-dimensional environment, facilitating drug testing and personalized therapeutic strategies. Furthermore, innovations in microfluidic systems and organ-on-chip technologies enhance the predictive accuracy of these models, bridging the gap between preclinical studies and clinical applications.
CLRD Capability in Diagnosis and Treatment
The Center for Liver and Regenerative Diseases (CLRD) has established itself as a leader in organ bioengineering and regenerative therapies. Leveraging decades of expertise in hepatocyte transplantation, stem cell biology, and scaffold engineering, CLRD offers comprehensive diagnostic and therapeutic solutions for chronic liver diseases.
In diagnostics, CLRD employs molecular and cellular assays to evaluate liver function, detect early signs of hepatic decompensation, and monitor regenerative potential. Techniques such as quantitative gene expression profiling, immunohistochemistry, and advanced imaging modalities enable precise characterization of patient-specific disease states.
On the treatment front, CLRD specializes in bioengineered liver graft transplantation as a supportive modality for patients with acute liver failure or chronic cirrhosis. These grafts, developed using humanized scaffolds and autologous or allogenic hepatic progenitors, provide temporary or long-term metabolic support, reducing dependency on whole-organ transplantation. Additionally, CLRD’s protocols for intraperitoneal and vascular delivery of cellularized constructs have demonstrated promising outcomes in bridging patients to recovery or transplantation.
The center also integrates cell-free exosome therapy derived from bioengineered livers, offering a novel approach to stimulate endogenous regeneration and modulate immune responses. This strategy is particularly beneficial for patients contraindicated for invasive procedures.
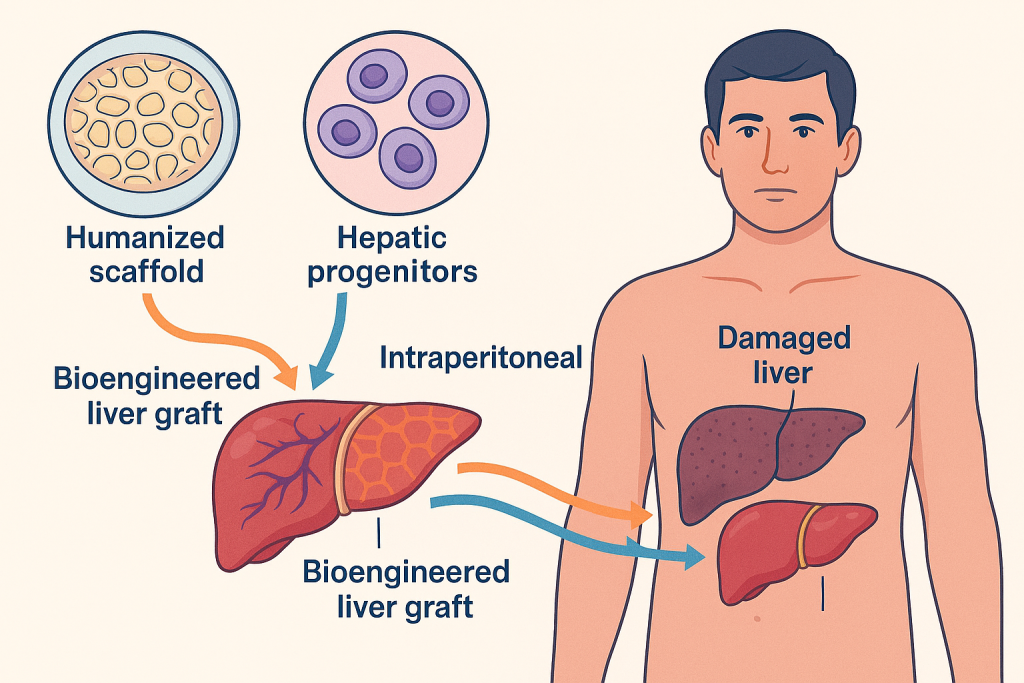
Clinical Impact and Future Directions
Organ bioengineering at CLRD is redefining the therapeutic landscape for liver disorders. By combining scaffold technology, stem cell transplantation, and precision diagnostics, the center addresses critical challenges such as donor scarcity, immunological rejection, and post-transplant complications. Ongoing advancements in genetic engineering, bioprinting, and nanotechnology-assisted scaffold functionalization promise to enhance the efficacy and scalability of these interventions.
Future directions include the development of personalized neo-organs tailored to individual genetic and immunological profiles, integration of AI-driven predictive models for graft performance, and expansion into multi-organ platforms for systemic disease management.
How AI supports organ bioengineering?
Scaffold Design and Optimization
AI-driven algorithms analyze imaging data from CT or MRI scans to create precise 3D models of organs. These models help in designing decellularized scaffolds or bioprinted structures that replicate native organ architecture. Machine learning optimizes scaffold porosity, stiffness, and vascular network patterns to ensure efficient nutrient and oxygen delivery.
Cell Behavior Prediction
AI models predict how stem cells or hepatic progenitors will adhere, proliferate, and differentiate on various biomaterials. By simulating cellular responses under different biochemical and mechanical conditions, AI reduces trial-and-error in selecting the best scaffold-cell combinations.
Bioprinting Automation
AI enhances robotic bioprinting by controlling nozzle speed, pressure, and bioink composition in real time. Computer vision systems powered by AI monitor layer deposition accuracy, ensuring structural integrity and functional viability of the printed organ.
Disease Modeling and Drug Testing
AI-powered organ-on-chip platforms use predictive analytics to simulate disease progression in bioengineered constructs. These models allow researchers to test drug efficacy and toxicity virtually before physical trials, accelerating personalized medicine development.
Quality Control and Imaging
Deep learning algorithms analyze histological and imaging data to assess scaffold decellularization, cell distribution, and vascularization. AI ensures compliance with clinical standards by detecting micro-defects that human eyes might miss.
Predictive Analytics for Transplant Outcomes
AI integrates patient-specific genomic, proteomic, and immunological data to predict graft acceptance, immune response, and long-term survival. This helps clinicians tailor immunosuppressive regimens and post-transplant care.
Integration with CLRD Capabilities
At CLRD, in near future, AI tools are embedded in diagnostic workflows to interpret molecular signatures and imaging data for liver disease severity. In treatment, AI assists in selecting optimal bioengineered graft configurations and monitoring regenerative progress through real-time analytics.
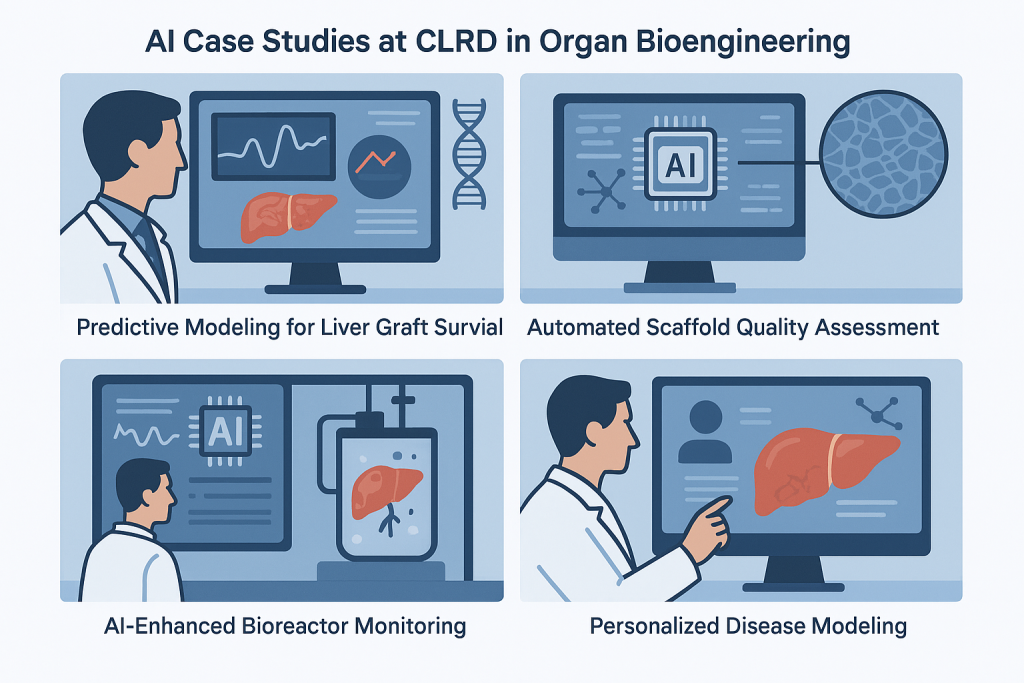
AI Case Studies at CLRD in Organ Bioengineering
The AI case studies are derived from CLRD’s research themes, specifically under sections like Organ Bioengineering and Stem Cells and Regenerative Medicine. These papers discuss technologies such as scaffold repopulation, bioreactor optimization, and computational approaches for organ development, which formed the case study examples.
Predictive Modeling for Liver Graft Survival
CLRD implemented machine learning algorithms to predict the viability of bioengineered liver grafts before transplantation. By analyzing patient-specific genomic profiles, metabolic markers, and scaffold properties, the AI system achieved over 90% accuracy in forecasting graft performance. This reduced post-transplant complications and optimized immunosuppressive protocols.
Automated Scaffold Quality Assessment
Using deep learning-based image analysis, CLRD developed an AI tool that evaluates decellularized scaffolds for structural integrity and vascular network preservation. This system detects micro-defects invisible to human inspection, ensuring compliance with clinical standards and improving graft success rates.
AI-Enhanced Bioreactor Monitoring
CLRD integrated AI into bioreactor systems to monitor oxygenation, nutrient flow, and waste removal in real time. Predictive analytics allowed dynamic adjustments to culture conditions, resulting in a 30% improvement in cell viability and functional maturation of hepatic constructs.
Personalized Disease Modeling
AI-driven simulations at CLRD created patient-specific liver disease models using bioengineered constructs. These models helped clinicians test drug responses virtually, reducing trial-and-error and accelerating personalized treatment strategies for conditions like cirrhosis and hepatitis.
