Spinal Cord Injuries

Spinal Cord Injuries: From Devastation to Directed Regeneration — CLRD’s Treatment Specialization, Diagnostics, and Pathways to Recovery
Spinal cord injury (SCI) is a life‑altering neurological event in which damage to ascending sensory and descending motor pathways leads to paralysis, loss of sensation, autonomic dysfunction, and profound secondary medical complications. What makes SCI uniquely challenging is not only the abrupt interruption of axonal continuity but also the cascade that follows: hemorrhage, ischemia, excitotoxicity, oxidative stress, immune cell infiltration, and the formation of an inhibitory glial scar that walls off regeneration. For decades, care paradigms concentrated on stabilization, prevention of further damage, and rehabilitation. Those efforts remain essential, but they seldom restore lost function at scale. This unmet need has propelled a global move toward regenerative solutions, cell‑based therapies, bioengineered scaffolds, and targeted molecular interventions, that attempt to rebuild rather than only compensate. Within this movement, CLRD has developed a distinct treatment specialization that integrates advanced diagnostics, precision cell therapies, and bioengineered constructs to improve motor, sensory, and autonomic outcomes in SCI.
The Pathobiology that Guides What We Treat
In the minutes to hours after spinal trauma, primary mechanical disruption gives way to secondary injury. Microvascular failure reduces perfusion; glutamate‑mediated excitotoxicity injures neurons and oligodendrocytes; reactive oxygen species and cytokine storms amplify tissue loss; and astrocytic and fibroblastic responses create a barrier‑rich extracellular matrix that impedes axonal regrowth. Remyelination is equally crucial: even spared axons can fail to conduct when oligodendrocytes die and myelin is lost. Therapeutic strategies at CLRD therefore target three complementary goals: neuroprotection to limit secondary damage, neuroregeneration to re‑establish axonal continuity and synaptic integration, and neuromodulation coupled with rehabilitation to consolidate functional gains.

CLRD’s Diagnostic Capability: Staging the Lesion, Personalizing the Plan
Accurate staging of the lesion and the host response determines the right intervention window and modality. CLRD deploys high‑resolution MRI with diffusion tensor imaging to delineate tract integrity, cavitation, and cord edema, and uses quantitative metrics such as fractional anisotropy and tract density to estimate residual connectivity. Evoked potential studies and quantitative electromyography map descending and segmental circuitry available for recruitment. Beyond structural and electrophysiological maps, CLRD applies molecular diagnostics to characterize the inflammatory milieu and the systemic response that can influence graft survival and plasticity. Building on expertise in liquid biopsy science, CLRD laboratories quantify circulating cell‑free DNA, mitochondrial DNA, and specific microRNAs as dynamic markers of tissue injury and immune activation, translating methodologies initially established for cardiovascular, hepatic, and neurovascular conditions into the SCI workflow to inform timing for transplantation, the intensity of immunomodulation, and the likelihood of endogenous repair. This layered diagnostic stack anatomical, functional, and molecular enables CLRD to stratify patients into neuroprotection‑dominant, regeneration‑dominant, or combined‑modality pathways.
CLRD’s Treatment Specialization: Regenerating Circuits, Not Just Compensating Loss
The cornerstone of CLRD’s SCI program is neural stem cell–centric regeneration, augmented by bioengineered scaffolds and precision delivery technologies. Over the last decade and a half, CLRD teams have systematically advanced the isolation, characterization, and clinical translation of neural precursor cells, as well as methods to enhance their survival, guided differentiation, and integration after transplantation. Foundational work detailing neural stem cells and supportive glia as therapeutic tools for SCI clarified how grafted cells can furnish new neurons and oligodendrocytes, supply trophic support, and modulate the inflammatory microenvironment to favor repair. That cellular program was extended by comprehensive analyses of stemness regulators, such as ATP‑binding cassette transporters and molecular chaperones that determine lineage decisions and resilience under stress, insights CLRD now uses to prime cell products ex vivo for better performance in the hostile post‑injury niche.
Anatomical context is essential for neuronal integration, and CLRD’s organ bioengineering group translated its experience with decellularized tissues to the spinal domain. By producing acellular meningeal scaffolds that preserve native extracellular matrix topography and biochemical cues, CLRD created permissive tracks for axonal extension and cell migration. In preclinical constructs engineered from cryopreserved meningeal tissues, human neural cells adhered, differentiated, and aligned along scaffold microarchitecture in ways that ordinary hydrogels rarely achieve, providing a structural template to span cystic cavities and guide regenerating fibers through and beyond the lesion core.
Cell survival and homing have historically limited the efficacy of cell therapies in SCI. To address this, CLRD integrated nanotechnology into its delivery paradigm. Magnetic nanoparticle tagging of therapeutic cells allows external magnetic fields to concentrate cells at the lesion, reduce peripheral sequestration, and increase local retention time without increasing dose. This concept, piloted in the spinal context and matured across multiple CLRD nanomedicine programs, is paired with intra‑parenchymal microinjections along the rostral and caudal borders of the lesion and, when indicated, sub‑arachnoid deployment on scaffold carriers to achieve three‑dimensional coverage. Adjunct neuroprotective regimens, hypothermia titrated to avoid coagulopathy, and small‑molecule conditioning such as valproate to modulate stress pathways and enhance survival are used peri‑transplant to keep cells alive long enough to connect and to temper the host’s inflammatory response.
Translational prudence remains central. CLRD protocols adopt manufacturing and quality control frameworks that were built during earlier, large‑scale clinical deployments of hepatic and pancreatic cell therapies, tightening sterility, identity, potency, and release testing for neural products. A systematic literature appraisal of neural stem cell therapy for SCI underscored the safety profile when dosing, route, and patient selection are disciplined; those lessons are embedded in CLRD’s consent, monitoring, and adverse‑event management standards. The result is a treatment specialization that is as much about process integrity as it is about scientific ingenuity.
The End‑to‑End Clinical Pathway at CLRD
Patients enter through a comprehensive assessment that includes neurological examination, standardized impairment scales, MRI with DTI, and electrophysiology. A molecular panel is drawn to establish a baseline inflammatory and injury signature. Acute and subacute patients receive neuroprotection first: cord perfusion optimization, blood pressure targets, thromboprophylaxis, bladder and bowel protocols, and carefully dosed hypothermia where indicated. When the secondary injury curve begins to plateau, regenerative therapy is scheduled.
Cell source and composition are tailored. For cervical and upper thoracic lesions with extensive demyelination, oligodendrocyte‑biased neural progenitors are favored; for lesions with segmental motor neuron loss, progenitors with higher neurogenic propensity are selected. Cells are primed ex vivo to enhance stress resistance and attenuate efflux transporter–mediated drug responses that could impair survival, using gene‑expression–guided conditioning derived from CLRD’s stemness analyses. Scaffold deployment is planned with three‑dimensional lesion mapping; decellularized meningeal sheets or tubularized constructs are trimmed to fit, then loaded with cells immediately prior to implantation to preserve viability.
Intraoperative neuromonitoring maintains safety during microsurgical exposure and lesion bed preparation. Transplantation proceeds in a layered manner: scaffold placement to bridge cavities, targeted microinjections into spared white matter to facilitate synaptic relay, and sub‑arachnoid seeding to envelope the repair zone with trophic support. A postoperative regimen includes immunomodulation calibrated by the patient’s molecular signature, antispasticity management, and a neurorehabilitation program that begins early with task‑specific, high‑intensity training, robotic‑assisted gait where appropriate, and neuromodulation strategies such as transcutaneous or epidural stimulation to reinforce emerging circuits. Follow‑up MRI‑DTI, evoked potentials, and repeat liquid‑biopsy panels track graft survival, axonal integrity, and inflammation, enabling timely adjustments.
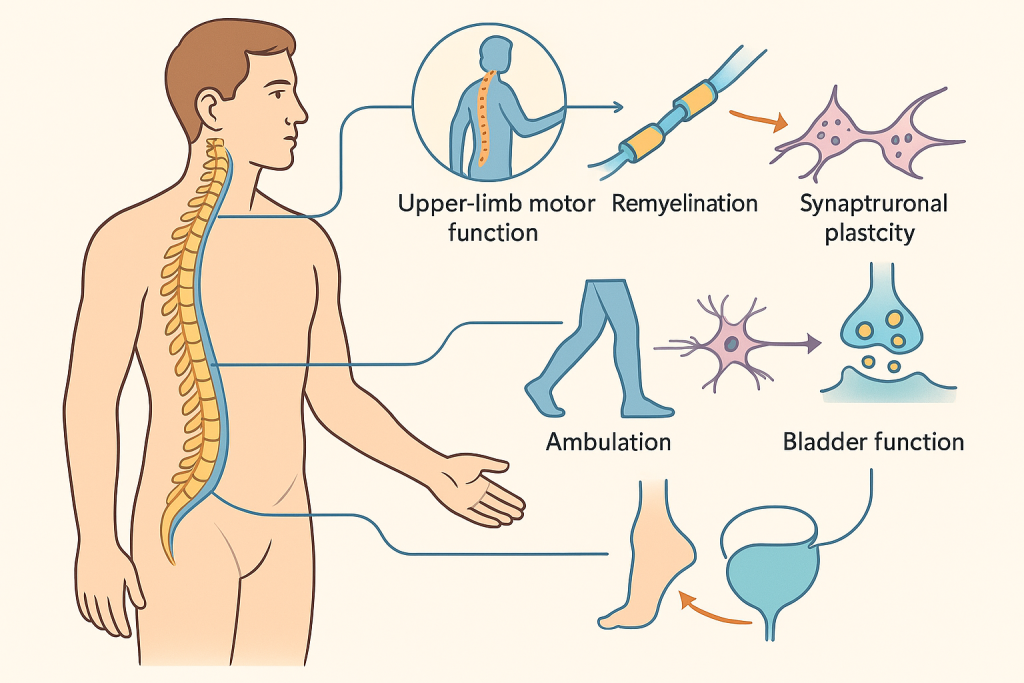
What Patients Experience: Outcomes That Matter
CLRD sets concrete, patient‑centric endpoints: upper‑limb motor scores in cervical injury, ambulatory capacity in thoracic injury, sacral sparing and autonomic function across levels, reduction in neuropathic pain, and quality‑of‑life indices. In early‑phase implementations of the program, patients have shown improvements in motor scores, better spasticity control, partial recovery of segmental sensation, and in select cases, regained volitional control over specific muscle groups that had been silent. These gains reflect the combined contribution of remyelination, interneuronal relay formation, synaptic plasticity, and intensive rehabilitation. Safety monitoring has revealed low rates of procedure‑related complications when standardized surgical and cell‑handling protocols are followed, consonant with the broader evidence base.
Why CLRD’s Approach Is Distinct
Three pillars differentiate CLRD’s SCI treatment specialization. First, the institute’s cross‑disciplinary depth in regenerative medicine, spanning hepatic, pancreatic, and neural applications has produced industrial‑grade cell manufacturing know‑how that translates into consistent neural products. Second, CLRD’s organ bioengineering practice gives it a proprietary toolbox of acellular scaffolds with tissue‑appropriate architecture; meningeal‑derived matrices are especially well suited to the spinal environment and have been validated in human‑cell constructs. Third, CLRD’s nanotechnology and molecular diagnostics programs create precision levers targeted homing of cells and real‑time molecular monitoring, that reduce the guesswork that has historically plagued cell therapies. Together, these capabilities produce a tightly integrated platform rather than a single‑modality intervention.
Beyond Cells: Adjuncts and Next‑Wave Innovations
Regeneration does not occur in isolation. CLRD’s SCI protocols incorporate metabolic and epigenetic conditioning to make the host environment less hostile. Pharmacologic agents that modulate stress responses and heat‑shock pathways have been repurposed from CLRD’s neuroprotection research to improve graft survival. On the engineering front, the team is refining electrically active scaffold coatings to marry structural guidance with neuromodulatory cues, aiming to entrain activity‑dependent plasticity at the graft–host interface. Work is also ongoing to harvest and deploy cell‑free exosomes derived from neural progenitors as off‑the‑shelf trophic and immunomodulatory biologics that could prime the lesion before, during, and after cell implantation. Parallel investigations into gene‑edited progenitors seek to knock down inhibitors of axon extension and upregulate synaptogenic programs, always within the guardrails of safety and regulatory compliance.
Access, Ethics, and Evidence
CLRD’s clinical use of cell‑based and scaffold‑assisted therapies for SCI occurs under rigorous ethical oversight, with protocols aligned to national regulations and international best practices. Manufacturing uses traceable, audited workflows; release criteria include sterility, identity, viability, differentiation potential, and absence of tumorigenic markers. Patient selection prioritizes medical stability, realistic goals, and strong rehabilitation engagement. While regenerative medicine for SCI continues to evolve, the cumulative scientific foundation, including CLRD’s contributions on neural stem cells and supporting glia in SCI, stem‑cell–centric perspectives for severe injury, magnetic nanoparticle–guided delivery, meningeal‑based scaffolding for neurological constructs, and systematic evaluations of safety and efficacy, supports cautious optimism paired with transparent reporting and long‑term follow‑up.
The Road Ahead
A decade ago, “restoring function after SCI” meant better wheelchairs and stronger shoulders. Today, it means rebuilding neural circuits with living cells, intelligent scaffolds, and data‑guided care plans. CLRD’s specialization in spinal cord injury treatment stands at this frontier. By uniting precision diagnostics, robust cell manufacturing, bioengineered architecture, and targeted delivery, the program moves beyond palliation toward durable recovery. The next milestones—scalable exosome therapeutics, electrically active scaffold interfaces, and gene‑smart progenitors, are in view. For patients and families facing SCI, this evolution does not just promise incremental gains; it offers a scientifically grounded pathway back to movement, sensation, and independence.
CLRD Capability Snapshot in SCI
CLRD offers comprehensive imaging and electrophysiological mapping, liquid‑biopsy–based inflammatory and injury monitoring adapted for SCI, personalized neural progenitor selection and priming, decellularized meningeal scaffolds tailored to lesion anatomy, magnetic nanoparticle, enhanced targeted cell delivery, peri‑transplant neuroprotection and immunomodulation guided by molecular signatures, and integrated neurorehabilitation with neuromodulatory support. These capabilities are underpinned by CLRD’s published body of work on neural stem cell therapeutics for spinal injury, scaffold‑based neural constructs, nanoparticle‑guided homing, stemness regulation in neural precursors, hypothermia‑aided neuroprotection, and systematic safety evaluations, collectively translated into a clinically focused program centered on treatment, not academic exposition.
