Degenerative diseases

Advanced Treatment Specialization for Parkinson’s Disease and ALS with CLRD Capabilities in Diagnosis and Therapy
Degenerative diseases of the nervous system demand lifelong, staged care that evolves as biology, symptoms, and personal priorities change. Parkinson’s disease and Amyotrophic Lateral Sclerosis (ALS) are emblematic of this challenge, each driven by distinct patterns of neuron loss yet both requiring carefully integrated programs that combine surgery, cellular therapy, and neuroprotective pharmacology. The Center for Liver and Regenerative Diseases (CLRD) delivers this integration as a treatment specialty, pairing precision diagnostics with interventional neurology, neurosurgery, and translational regenerative medicine. The approach emphasizes rigorous phenotyping at baseline, targeted interventions that are escalated or de‑escalated over time, and systematic use of cellular and molecular insights to slow degeneration, sustain function, and preserve quality of life.
Parkinson’s Disease: Treatment Specialization Built on Staged, Personalized Intervention
Parkinson’s disease results primarily from progressive degeneration of dopaminergic neurons within the substantia nigra and their projections to the striatum. The clinical expression, bradykinesia with either rigidity, rest tremor, or postural instability, unfolds across years, and the therapeutic arc must anticipate motor fluctuations, dyskinesias, and non‑motor burdens such as cognitive and autonomic change. CLRD’s program begins with a precision diagnostic workup that stratifies patients by motor phenotype, cognitive baseline, autonomic involvement, and comorbid conditions that affect device therapy and advanced pharmacology. Beyond neurological examination, the diagnostic framework incorporates quantitative movement assessment, objective tremor and bradykinesia metrics, and advanced laboratory profiling. Molecular diagnostics,an institutional strength, include circulating nucleic acid and microRNA workflows validated across CLRD’s broader translational portfolio, mitochondrial integrity assessments adapted from platelet and plasma assays the institute has used in other disease contexts, and inflammation and stress response panels derived from CLRD’s extensive gene expression and immunology expertise. These methods, developed and refined through years of CLRD-led transcriptomic and stress‑response research, allow clinicians to align neuroprotective strategies with individual biological signatures rather than relying solely on clinical staging.
The pharmacological foundation of care emphasizes early optimization of dopaminergic tone while protecting long‑term neural viability. Initial regimens are calibrated to delay motor complications where feasible and to minimize off‑time once fluctuations emerge. CLRD pairs dopaminergic therapies with neuroprotective strategies designed to mitigate oxidative stress and proteostatic strain, drawing on internal know‑how around heat‑shock protein modulation, ATP‑binding cassette transporter biology, and cellular stress pathways that the institute has investigated in human neural precursor cells and related models. In practice, this translates into structured use of antioxidant and mitochondrial‑supportive agents, careful titration of glutamatergic modulators in appropriate patients, metabolic optimization, and gut–brain axis management aimed at reducing systemic inflammatory drivers that can accelerate nigrostriatal vulnerability. Patients are monitored against individualized molecular and functional baselines, and therapy is iteratively adjusted to maintain target exposure while limiting cumulative toxicity.
Surgical therapy, led by a dedicated functional neurosurgery team, provides a durable solution for medication‑refractory tremor, dyskinesias driven by pulsatile dopamine delivery, and severe off‑period disability. Deep Brain Stimulation is offered following standardized candidacy evaluation that includes motor diaries, levodopa challenge quantification, cognitive screening to preserve safety and outcomes, and neuroimaging for target selection. Intraoperative and postoperative programming protocols at CLRD leverage quantitative kinematic feedback and longitudinal device analytics to stabilize motor output while sustaining speech and gait function. For patients unsuitable for DBS, CLRD offers advanced infusion therapies and ablative options, integrated with the same outcomes‑tracking framework to ensure continuity of care across modalities.
Cellular therapy is the signature differentiator of CLRD’s Parkinson’s care pathway. Building on decades of stem cell science that encompasses neural precursors, hepatic progenitors, mesenchymal stromal cells, and exosome‑based signaling, the program deploys cell‑derived interventions as adjuncts to standard care in defined clinical scenarios. CLRD’s teams have published on neural stem cells and supportive glia as therapeutic tools in central nervous system injury, on isolation and characterization of human fetal brain neural precursors, and on the molecular programs, such as ABC transporters and chaperones, that govern stemness and survival in human neural precursor cells. These capabilities translate into two practical treatment avenues. The first is intracerebral cell placement for dopaminergic circuit support in carefully selected patients, using cells characterized for lineage potential and neurotrophic profiles. The second is the administration of cell‑free biologics, particularly exosomes engineered or selected for neuroprotective cargo, a strategy informed by CLRD’s leadership in bioengineered, cell‑free regenerative products within hepatology that are now being adapted to neurology. In parallel, CLRD’s nanotechnology and drug‑delivery expertise enables targeted conveyance of neuroprotective agents to deep brain structures with controlled release, enhancing local efficacy while minimizing systemic exposure.
The non‑motor dimensions of Parkinson’s disease receive equal emphasis because they are decisive for independence and wellbeing. Autonomic dysfunction is addressed through cardiovascular and gastrointestinal co‑management; sleep and mood disorders are treated within a neuropsychiatry workflow designed to avoid exacerbating motor symptoms; and cognitive change is tracked with repeatable digital and in‑person instruments. Rehabilitation is integrated from diagnosis onward, with cueing strategies, amplitude‑focused movement training, speech therapy, and adaptive cognitive rehabilitation. CLRD’s care coordination model assigns a navigator to each patient, ensuring uninterrupted transitions between medication optimization, device therapy, cellular or exosome‑based adjuncts, and supportive care, while continuously aligning treatment with patient‑defined goals.
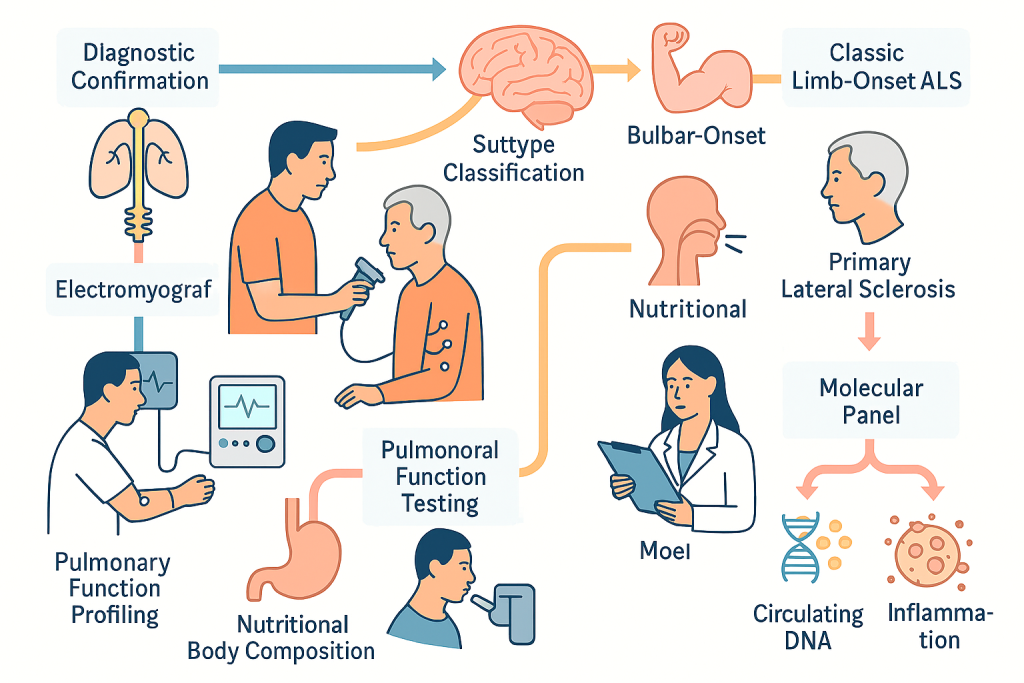
Amyotrophic Lateral Sclerosis (ALS): Multimodal Care that Combines Disease Modification, Function Preservation, and Systems‑Level Support
ALS is characterized by the degeneration of upper and lower motor neurons, producing progressive weakness, atrophy, spasticity, bulbar dysfunction, and eventual respiratory failure. Because the disease course is heterogeneous, CLRD’s approach begins with a rigorous diagnostic confirmation and subtype classification that distinguishes classic limb‑onset ALS from bulbar‑onset and primary lateral sclerosis patterns, accounts for frontotemporal cognitive involvement, and rules out mimics. The baseline data set includes electromyography with objective motor unit analysis, pulmonary function and nocturnal gas exchange testing, nutritional status and body composition profiling, and a molecular panel focused on neuronal stress and inflammation. While ALS lacks a single definitive biomarker, CLRD adapts its validated circulating DNA, mitochondrial function, and RNA‑based workflows, developed across cardiovascular, immunology, and neuro‑inflammatory research programs—to construct individualized reference points for disease activity and to monitor the biological impact of therapy.
Disease‑modifying pharmacology is instituted early and layered rationally. Agents targeting glutamate excitotoxicity, oxidative injury, and RNA metabolism are combined where indicated, with a structured plan to escalate or switch based on tolerance, progression rate, and biomarker movement. CLRD’s pharmacology unit leverages nanocarrier and conjugate systems, pioneered in oncology and hepatology, to improve central nervous system penetration and sustain exposure, thereby extracting more benefit from existing drugs. Symptomatic therapy is mapped to the major drivers of disability: spasticity, cramps, sialorrhea, pseudobulbar affect, dysphagia, and dyspnea. Respiratory care follows proactive thresholds for initiation of non‑invasive ventilation and cough augmentation, timed to protect sleep architecture and daytime function; gastrostomy is planned before critical weight loss or aspiration risk spiral.
Surgical interventions in ALS are predominantly supportive yet crucial for survival and comfort. Tracheostomy for long‑term ventilation and percutaneous endoscopic gastrostomy for nutrition are delivered within a values‑based decision framework that provides patients and families with clear projections of function, burden, and quality of life under different scenarios. Orthopedic and spasticity procedures are considered in select cases to preserve positioning, hygiene, and ease of care. CLRD’s perioperative protocols are adapted to neuromuscular vulnerability, with respiratory and anesthesia teams experienced in ALS physiology ensuring safe procedural courses.
Cellular therapy is strategically developed at CLRD as an adjunct to standard ALS care. The institute’s stem cell programs, in neural, hepatic, and mesenchymal lineages, as well as organ bioengineering, inform the selection, characterization, and delivery of cells or cell‑derived biologics designed to modulate the motor neuron microenvironment. Preclinical work on neural precursors, combined with experience isolating and expanding human neural cells and analyzing their chaperone and transporter programs, supports two categories of intervention. Intrathecal delivery of trophic factor‑rich mesenchymal derivatives seeks to dampen neuroinflammation and support motor neuron survival across the neuraxis, while regionally targeted placement of neural lineage cells aims to buttress motor pools most responsible for respiratory and bulbar function. As with Parkinson’s disease, exosome‑based therapeutics are under active translation, drawing from CLRD’s established protocols for generating and quality‑controlling cell‑free products in other organ systems. Each cellular or exosomal intervention is embedded in a monitoring protocol that uses clinical scales, respiratory metrics, and individualized molecular panels to detect biological signal early and to decide on repeat dosing cadence.
Rehabilitation and assistive technology at CLRD are delivered as a continuous, anticipatory service rather than a series of late responses. Physical and occupational therapy focus on energy‑efficient movement, joint preservation, and task substitution before weakness crosses functional thresholds. Communication is preserved through staged introduction of speech‑generating devices and eye‑tracking systems, with early training to avoid abrupt transitions. Nutrition is treated as disease modification, using targeted macronutrient strategies and texture management to sustain weight and reduce metabolic stress. Palliative care is introduced at diagnosis to align decisions with personal values, reduce symptom burden, and coordinate home‑based services; it remains a partner to disease‑modifying therapy rather than a replacement.
CLRD Diagnostic and Therapeutic Capabilities: What Distinguishes the Care Pathway
The diagnostic platform leverages CLRD’s track record in circulating nucleic acids and mitochondrial biology. Assays for cell‑free DNA and mitochondrial DNA, originally optimized in cardiovascular, sepsis, and stroke contexts, are adapted to neurodegeneration to provide non‑invasive readouts of cellular injury and systemic stress. Gene‑expression meta‑analytic approaches refined in autoimmune and inflammatory diseases are repurposed to construct compact biomarker panels relevant to neuronal stress, proteostasis, and neuroinflammation. In practical terms, this means patients with Parkinson’s disease and ALS at CLRD receive baselines that go beyond clinical staging, enabling clinicians to time escalations, select candidates for cellular or exosomal therapy, and quantify biological response rather than relying solely on functional scores.
Surgically, CLRD’s functional neurosurgery and neuromuscular procedural teams apply precision imaging, intraoperative physiology, and structured post‑operative programming to deliver deep brain stimulation, intrathecal therapies, and supportive procedures with reproducible outcomes. Device‑based interventions are embedded within a longitudinal analytics program that captures kinematics, speech and gait features, and patient‑reported outcomes to guide parameter updates and to confirm durable benefit.
The pharmacology and delivery science units at CLRD adapt nanocarriers, conjugates, and advanced substrates, platforms first validated in hepatology and oncology, to increase central nervous system exposure and target engagement for neuroprotective agents. Parallel expertise in decellularized scaffolds and tissue‑mimetic constructs informs microenvironment‑aware drug and cell delivery to neural tissues, a practical advantage when translating regenerative strategies that depend on niche support.
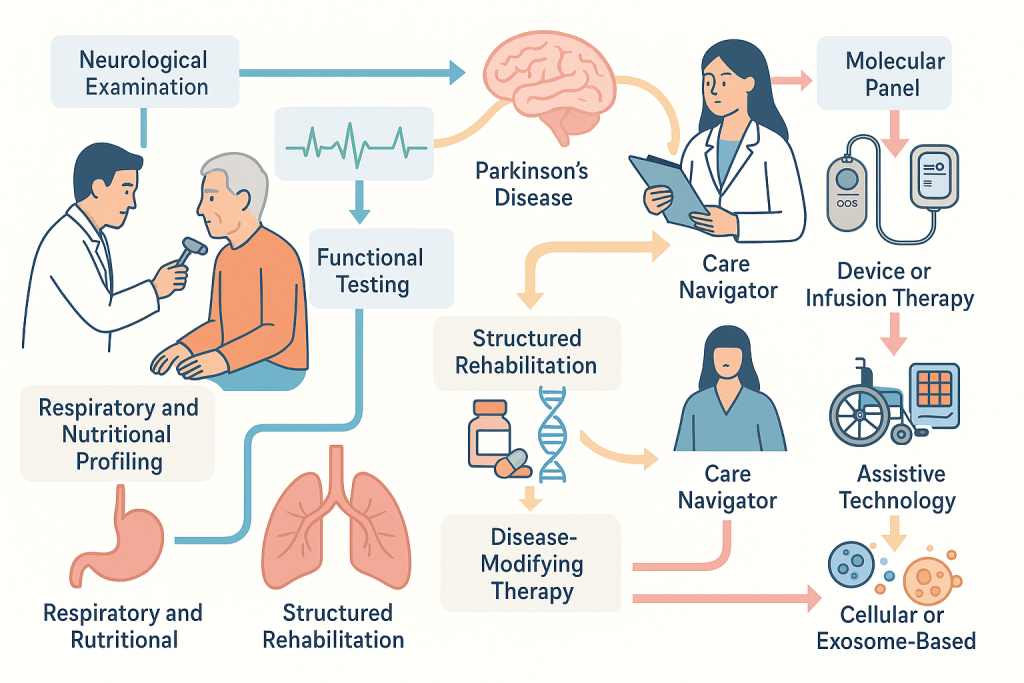
The Care Experience: How Patients Move Through the Program
Patients enter through a comprehensive consult that synthesizes neurological examination, functional testing, respiratory and nutritional profiling where applicable, and a tailored molecular panel. A written plan outlines short‑term stabilization and long‑term staging of interventions. For Parkinson’s disease, this often begins with pharmacological optimization and structured rehabilitation, followed by candidacy evaluation for device or infusion therapy and discussion of cellular or exosome‑based adjuncts. For ALS, disease‑modifying therapy begins immediately alongside a proactive respiratory and nutrition plan, with timelines for assistive technology adoption and criteria for cellular or exosomal interventions. In both conditions, a care navigator coordinates appointments, device programming, therapy cycles, and home‑based services, while multidisciplinary case conferences recalibrate strategy as biology and function evolve.
Outcomes are measured against individualized anchors: symptom targets, off‑time and dyskinesia windows in Parkinson’s disease, respiratory milestones and communication independence in ALS, and molecular biomarkers that indicate whether neuroprotective or cellular strategies are producing the intended biological shift. When the data indicate insufficient benefit, CLRD escalates or pivots, including enrollment into treatment protocols that extend access to emerging cellular and cell‑free products.
Exosome-Based Therapies: A New Frontier in Neuroregeneration
Exosomes-nano-sized extracellular vesicles secreted by cells, are emerging as powerful mediators of intercellular communication and tissue repair. Unlike whole-cell transplantation, exosome-based therapy delivers bioactive molecules such as proteins, lipids, and microRNAs directly to target cells, bypassing many immunological and logistical challenges associated with live cell grafts. These vesicles can cross the blood–brain barrier, making them particularly suited for neurodegenerative conditions like Parkinson’s disease and ALS.
At CLRD, exosome therapeutics are developed from rigorously characterized stem cell populations, ensuring consistency in cargo composition and potency. Leveraging decades of expertise in stem cell biology and regenerative medicine, CLRD engineers exosomes to carry neuroprotective factors, anti-inflammatory signals, and mitochondrial stabilizers. Delivery strategies include intrathecal administration for ALS and targeted intracerebral infusion for Parkinson’s disease, supported by CLRD’s nanotechnology platforms that enhance localization and controlled release.
The advantages of exosome therapy are manifold:
- Safety: No risk of uncontrolled cell proliferation or tumorigenesis.
- Scalability: Easier manufacturing and storage compared to whole-cell therapies.
- Precision: Ability to customize cargo for disease-specific pathways, such as dopaminergic neuron survival in Parkinson’s or motor neuron resilience in ALS.
CLRD’s translational pipeline integrates exosome therapy into its multimodal care framework, offering patients access to cutting-edge interventions that complement pharmacology, surgery, and rehabilitation.
CLRD’s Commitment to Transformative Care
Degenerative diseases like Parkinson’s and ALS demand more than symptomatic relief—they require a paradigm that combines molecular insight, regenerative innovation, and patient-centered care. CLRD stands at the forefront of this transformation, uniting advanced diagnostics, surgical precision, cellular and exosome-based therapies, and neuroprotective pharmacology into a cohesive treatment ecosystem. By bridging bench-to-bedside science with clinical excellence, CLRD delivers not only hope but measurable progress for patients facing these relentless conditions.
The future of neurodegenerative disease management lies in integration—where biology, technology, and compassion converge. At CLRD, that future is already unfolding.
Why CLRD for Degenerative Disease Treatment
CLRD’s distinguishing strength is the union of advanced clinical services with a mature regenerative medicine engine. Neural stem cell expertise, demonstrated isolation and characterization of human neural precursors, deep understanding of transporter and chaperone biology in neural stemness, validated strategies for neuroprotection under cellular stress, and a long history of clinical‑grade cell therapy in other organ systems collectively shorten the path from concept to clinic in Parkinson’s disease and ALS. The same ecosystem that produced clinical hepatocyte and hepatic progenitor cell therapies, bioengineered tissues, and cell‑free exosomes now powers neurology‑specific cellular and exosomal interventions. Patients thereby receive not only the current standard of care, precise pharmacology, best‑practice surgery, and comprehensive supportive care, but also access to carefully developed regenerative adjuncts supported by quality systems, analytics, and follow‑up frameworks that are already part of CLRD’s DNA.
Parkinson’s disease and ALS require more than a set of prescriptions; they demand a living plan that matches the biology and the person, revisited constantly and executed by a team with the tools to pivot as the disease changes. CLRD delivers that plan through staged pharmacology, best‑in‑class surgical options, and a regenerative program that deploys cells and cell‑free products with scientific discipline. Diagnostics extend beyond labels to molecular and mitochondrial signatures that guide timing and selection of advanced therapies. Rehabilitation, technology, and palliative partnership are woven into the fabric of care from day one. The result is a treatment specialization that offers patients and families a coherent path forward, one that protects function now while investing in the neuronal resilience needed for the years ahead.
