Neuro‑Liver Axis & Brain–Liver Care
Exploring brain–liver connections to develop neuroprotective therapies and regenerative solutions for liver‑related neural disorders
The neuro‑liver axis describes the tightly regulated, bidirectional crosstalk between hepatic function and the central nervous system through immune, metabolic, autonomic, endocrine, and microbiome‑derived signals. When the liver is inflamed, cirrhotic, or acutely failing, ammonia, bile acids, manganese, inflammatory cytokines, and pathogen‑associated molecules escape normal detoxification, alter blood–brain barrier transporters and astrocyte homeostasis, and trigger neuroinflammation and cerebral edema leading to cognitive and motor impairment classically seen in hepatic encephalopathy. Conversely, brain injury and neurodegeneration can dysregulate hepatic glucose and lipid metabolism via autonomic efferents and circulating stress mediators, worsening systemic inflammation and hepatic vulnerability. At CLRD, this systems view underpins an integrated clinical program that couples rapid toxin control and ammonia detoxification with neuroprotection, immunomodulation, and liver regeneration to restore cross‑organ homeostasis, drawing on decades of translational hepatology, stem‑cell therapy, bioartificial organ engineering, neural repair, and liquid‑biopsy innovation published by our teams.
Conditions we focus on across the neuro‑liver axis
Our treatment specialization spans acute and chronic hepatic encephalopathy in cirrhosis, acute liver failure with cerebral edema risk, post‑transplant neurocognitive syndromes, and neuro‑metabolic consequences of cholestatic and inherited hepatic disorders. We also manage brain injury states that secondarily compromise hepatic function, including sepsis‑associated encephalopathy with concurrent liver injury and neuroinflammation, where our immune‑signature and mitochondrial‑damage biomarker work informs care escalation.

Pathobiology and therapeutic leverage points
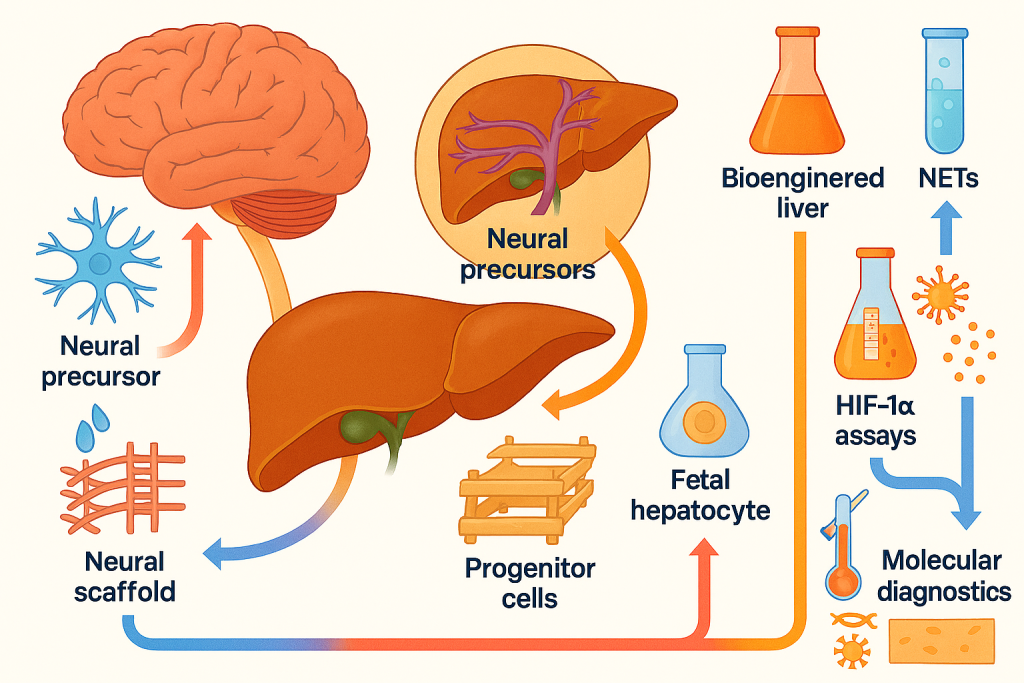
In hepatic encephalopathy, excess ammonia is converted to glutamine within astrocytes causing osmotic swelling, while bile acids and manganese disturb neurotransmission and mitochondrial respiration. Microglial priming by circulating cytokines sustains neuroinflammation and synaptic dysfunction. Our program targets four leverage points: accelerated hepatic support to reduce toxin load; restoration of ammonia handling via hepatocyte and progenitor‑cell–based regeneration; direct neuroprotection to stabilize astrocyte–neuronal networks; and immune rebalancing to quell microglial activation. These pillars are rooted in CLRD’s pioneering human fetal hepatocyte and hepatic progenitor transplantation in liver failure and hyperbilirubinemic states, our bioengineered humanized liver grafts and exosome‑based liver‑support concepts, and our neural stem‑cell and neuroprotectant investigations, all of which provide a mechanistic basis to modulate both sides of the axis.
CLRD diagnostic capability for neuro‑liver disorders
We deliver a layered diagnostic pathway that begins with rapid assessment of precipitating factors and severity scoring, coupled with advanced ammonia quantification and bile‑acid profiling. Neuroimaging and neurophysiology are integrated early for patients with altered sensorium, using MRI to evaluate manganese deposition or white‑matter edema and EEG to grade encephalopathy, while our laboratory quantifies systemic inflammatory and endothelial‑injury signals that track brain risk in liver disease. Building on our meta‑analyses and original work identifying immune expression signatures and stress‑response pathways in inflammatory states, we incorporate transcript‑ and protein‑level markers when encephalopathy is disproportionate to ammonia or when sepsis is suspected. Our liquid‑biopsy portfolio extends to cell‑free nuclear and mitochondrial DNA, neutrophil extracellular traps, and hypoxia pathways, which we have developed and applied in cerebrovascular and critical‑illness contexts and now translate to neuro‑liver cases for real‑time risk stratification and monitoring.

CLRD treatment specialization: comprehensive care pathways
Our core clinical pathway starts with stabilization and rapid toxin control. We institute goal‑directed ammonia reduction using optimized lactulose and rifaximin protocols, tailored nutrition that achieves adequate protein without exacerbating ammoniagenesis, microbiome‑targeted adjuncts, and early correction of precipitants such as GI bleeding, infection, electrolyte shifts, and constipation. In hyperacute settings with grade III–IV encephalopathy or refractory hyperammonemia, we escalate to continuous renal‑replacement strategies or molecular adsorbent recirculating systems as bridges to hepatic recovery or regenerative therapies. These steps are complemented by neuroprotective measures that we have validated preclinically leveraging hypothermia paradigms and valproate‑linked cytoprotective pathways in human neural precursors to attenuate heat‑shock and transporter dysregulation observed in neural injury models, with protocolized temperature control, sedation strategies, and seizure prophylaxis individualized to encephalopathy grade and intracranial pressure risk.
When medical therapy plateaus, CLRD’s hallmark is regenerative support. We evaluate candidacy for hepatic progenitor and stem‑cell–based interventions, an area where our group has led first‑in‑human applications of hepatic stem/progenitor transplantation in cirrhosis and metabolic disorders, demonstrated safety of autologous marrow‑derived cell infusions through the hepatic artery for chronic liver failure, and developed methods for in vivo tracking and immunologic monitoring of transplanted cells. For acute liver failure with neurological compromise, we consider intraperitoneal or intra‑arterial delivery of hepatocytes or progenitors to provide ammonia detoxification and synthetic function support while the native liver recovers. Our bioengineered humanized neo‑liver constructs and decellularized scaffolds expand options for patients who are poor transplant candidates or face long wait‑times, offering a supportive bridge that reduces neurotoxin burden and stabilizes cognition.
In parallel, we address brain‑forward mechanisms. For patients with overlapping neurodegeneration, spinal cord injury, or cerebrovascular injury complicated by liver dysfunction, our neural stem‑cell and neural‑construct platforms inform rehabilitation‑linked trials. We have engineered meningeal‑derived acellular scaffolds and neural constructs to promote axonal regrowth, and we integrate these insights into multidisciplinary neurorehabilitation for post‑encephalopathy cognitive and motor recovery. Our program also screens for and treats sleep wake inversion, sarcopenia, and frailty with targeted exercise, branched‑chain amino acids, and circadian hygiene to reduce encephalopathy recurrence, guided by transporter and heat‑shock responses we have characterized in human neural cells under stress.
Precision monitoring and follow‑up
Clinical improvement is validated against quantitative milestones: resolution of asterixis and attentional deficits, ammonia and bile‑acid normalization, inflammatory and endothelial biomarker down‑trends, and reduction of circulating cell‑free mitochondrial DNA and NETs when sepsis has complicated the course. Our teams have standardized simultaneous extraction and quantification protocols for nuclear and mitochondrial cfDNA from single plasma draws, enabling practical incorporation into high‑acuity rounds, while stroke‑program methodologies for cfDNA and hypoxia indices are adapted to hepatic encephalopathy follow‑up where white‑matter injury is suspected. We complement labs with computerized cognitive testing and caregiver‑reported functional scales to ensure that biochemical recovery matches real‑world neurocognition, and we debrief precipitant‑prevention bundles to minimize readmission.
Advanced and interventional options we offer
For select patients with recurrent, refractory encephalopathy despite maximal medical therapy, we provide consultation on shunt modification when portosystemic flow is excessive and screen aggressively for covert seizures and spontaneous bacterial peritonitis with rapid, protocol‑driven management. Patients with metabolic or cholestatic etiologies access our clinical trials that repurpose hepatic progenitor cells toward bile‑acid homeostasis and ammonia cycling, informed by our lineage‑tracing, enzyme‑activity, and gene‑transfer experience in hepatocyte biology and by our development of humanized liver platforms for drug testing. Where immune dysregulation dominates, we individualize short immunomodulatory courses based on integrated stress‑response and inflammasome signatures published by our investigators, monitoring closely with our transcript/protein panels to avoid overt immunosuppression.
How CLRD’s research translates to bedside care
CLRD has a sustained record at the frontiers of liver regeneration first demonstrating human fetal hepatocyte transplantation in fulminant hepatic failure, then advancing hepatic progenitor cell therapy for cirrhosis and metabolic hyperbilirubinemia, establishing safety of cell infusions via hepatic artery, and innovating bioengineered humanized livers and exosome‑based liver support that together lay the foundation for practical, scalable bridges to transplant or recovery. In parallel, our neural sciences contributions encompass isolation and characterization of human neural precursors, neuroprotective conditioning against ethanol‑mediated and heat‑stress injury, and construction of neural scaffolds for repair tools we now deploy to mitigate brain injury during liver crises and to accelerate neurocognitive rehabilitation afterward. Our immunology and liquid‑biopsy portfolio, including cfDNA, NETs, HIF‑1α assays, microRNA diagnostics, and endothelial‑injury markers, equips our intensive care and step‑down units with real‑time molecular telemetry of brain–liver risk that complements clinical examination and imaging. This full bench‑to‑bedside arc is unique in allowing us to intervene early, support failing systems, and verify recovery at the molecular and functional levels.
What patients and referring clinicians can expect
Patients are admitted to a coordinated neuro‑hepatic service line. Day 0 priorities are airway protection when indicated, precipitant reversal, ammonia‑lowering, electrolyte stewardship, early neuroimaging and EEG for severe grades, and institution of our neuroprotective bundle. By 24–48 hours we layer precision monitoring, including liquid‑biopsy panels where appropriate, and determine candidacy for regenerative support. From Day 3 onward we advance nutrition, mobilization, and cognitive rehabilitation, with transplant or cell‑based support decisions finalized by a multidisciplinary board. Upon discharge, patients transition to a prevention clinic with titrated rifaximin/lactulose, sarcopenia management, microbiome‑sensitive diet, and scheduled cognitive follow‑ups, while caregivers receive tailored education and direct access to our rapid‑response team to pre‑empt decompensation. This pathway has been iteratively refined from our translational portfolio across hepatocyte biology, stem‑cell therapy, organ bioengineering, neural repair, and molecular monitoring to maximize safety and functional outcomes.
Why CLRD for neuro‑liver care
CLRD combines seminal first‑in‑human hepatic cell therapies, cutting‑edge bioengineered organ supports, robust neural regeneration expertise, and validated molecular diagnostics under one coordinated program a rare integration that directly targets the biology of the neuro‑liver axis. Our hepatology, neurology, intensive care, interventional, and rehabilitation teams operate on shared protocols matured through our publications, ensuring that every patient benefits from solutions already vetted in our labs and early clinical studies. For referring clinicians, this means a partner capable of stabilizing the brain while regenerating the liver and of measuring success with tools sensitive enough to detect relapse before symptoms recur.
