Autoimmune Liver Conditions
Why CLRD focuses on autoimmune liver diseases
The Center for Liver Research & Diagnostics (CLRD) has built a translational pipeline that spans discovery immunology, molecular diagnostics, and regenerative hepatology. Over decades, the team has accumulated deep expertise in
- immune dysregulation biology,
- non‑invasive biomarker development (including circulating cell‑free DNA and neutrophil extracellular trap (NET) signatures), and
- advanced liver cell therapeutics and bioengineered models.
Together, these strengths position CLRD to tackle complex autoimmune liver conditions specifically Autoimmune Hepatitis (AIH) and Primary Biliary Cholangitis (PBC) with precision tools for identifying autoreactive antibodies, profiling immune and endothelial dysfunction markers, and real‑time disease monitoring.
About Autoimmune Liver Diseases
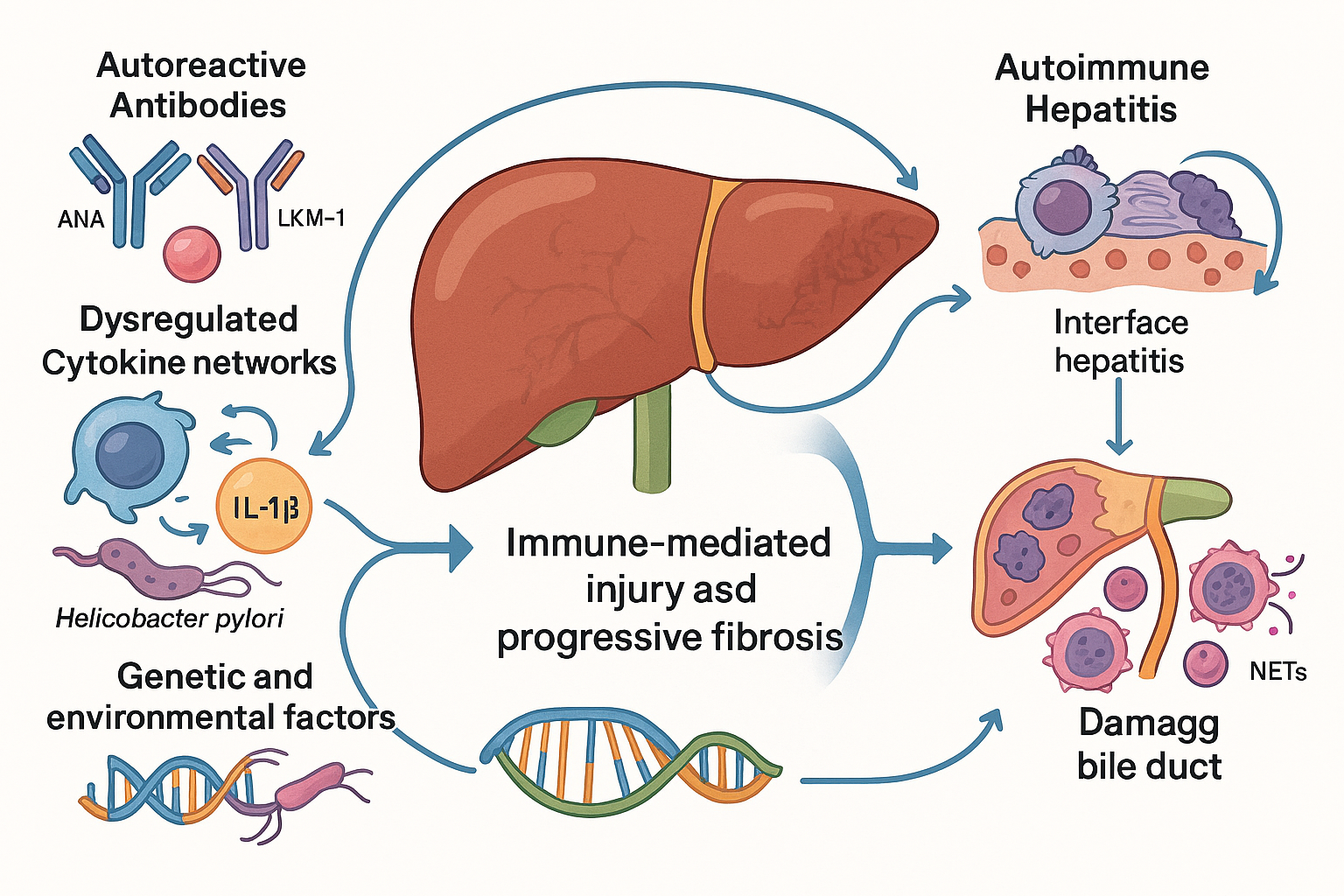
Autoimmune liver diseases constitute a distinct group of chronic hepatic disorders in which the immune system erroneously targets the liver’s own cells and structures. Unlike infectious or metabolic liver conditions, these diseases are driven by complex immunological mechanisms that involve autoreactive antibodies, dysregulated cytokine networks, and genetic predispositions interacting with environmental triggers. The two most clinically significant entities in this category are Autoimmune Hepatitis (AIH) and Primary Biliary Cholangitis (PBC), each with unique pathological features yet sharing common themes of immune-mediated injury and progressive fibrosis.
Autoimmune Hepatitis: Nature and Complexity
Autoimmune Hepatitis is a chronic inflammatory condition characterized by hepatocellular damage resulting from an aberrant immune response. Histologically, AIH is marked by interface hepatitis, a pattern where lymphoplasmacytic infiltrates breach the limiting plate and invade hepatic parenchyma. Biochemically, patients exhibit elevated aminotransferases and hypergammaglobulinemia, reflecting ongoing immune activation. Serologically, AIH is defined by disease-specific autoantibodies such as ANA, SMA, and LKM-1, which serve as diagnostic hallmarks. However, the disease does not present uniformly; its onset can be insidious or acute, and its progression varies widely among individuals. Genetic factors, including HLA associations, and environmental influences such as viral infections or drug exposures, modulate susceptibility and clinical course. This heterogeneity complicates early diagnosis and necessitates a more nuanced approach to disease monitoring and management.
Primary Biliary Cholangitis: A Cholestatic Autoimmune Disorder
Primary Biliary Cholangitis represents another facet of autoimmune liver pathology, primarily targeting the small intrahepatic bile ducts. The immune-mediated destruction of these ducts leads to impaired bile flow, chronic cholestasis, and eventual hepatic fibrosis. Clinically, PBC often begins with subtle biochemical changes, such as elevated alkaline phosphatase, before progressing to symptomatic stages marked by fatigue, pruritus, and complications of portal hypertension. The presence of antimitochondrial antibodies (AMA) remains the most specific serological marker, yet the disease involves a broader immunological landscape, including T-cell mediated injury and inflammatory cytokine cascades. Like AIH, PBC exhibits variable trajectories, with some patients experiencing slow progression while others advance rapidly to cirrhosis and liver failure.
Why Precision is Imperative
The clinical heterogeneity of AIH and PBC underscores the need for precision in diagnosis and management. Conventional approaches rely heavily on static serological markers and periodic liver biopsies, which provide only snapshots of disease activity. These methods often fail to capture dynamic fluctuations between remission and flare, leaving clinicians without timely indicators for therapeutic adjustment. Furthermore, reliance on invasive procedures such as biopsy introduces risks and patient discomfort, limiting their utility for frequent monitoring.
CLRD addresses these challenges through a research-driven, biomarker-first strategy that integrates traditional diagnostics with advanced molecular tools. By leveraging circulating signals such as neutrophil extracellular traps and cell-free DNA, CLRD enables real-time assessment of immune activity and tissue injury. These non-invasive biomarkers complement histology and serology, offering continuous visibility into disease dynamics and facilitating response-guided therapy. This approach transforms autoimmune liver disease management from episodic evaluation to proactive, data-informed care, ensuring earlier intervention, optimized immunosuppression, and improved long-term outcomes.

CLRD’s Research‑Enabled Capabilities for Autoimmune Liver Conditions
1) Identifying autoreactive antibodies & immune dysregulation markers
One of the most critical aspects of understanding autoimmune liver diseases lies in the ability to accurately identify autoreactive antibodies and the broader spectrum of immune dysregulation markers. CLRD has built a strong foundation in this domain through decades of research that bridges immunology and clinical hepatology. Historically, the center pioneered methods for detecting autoreactive antibodies and circulating immune complexes in immune-mediated conditions and infection-linked autoimmunity. These early innovations established assay frameworks that are now being adapted and refined for autoimmune hepatitis and primary biliary cholangitis, enabling enhanced serological profiling beyond conventional markers.
The complexity of autoimmune liver diseases extends beyond antibody detection. CLRD’s research has delved into innate and adaptive immune signatures, uncovering novel molecular patterns through meta-analyses and transcriptomic studies. Investigations into systemic autoimmune disorders such as rheumatoid arthritis and ulcerative colitis revealed critical pathways involving RNA-binding proteins and stress-response regulators. These findings inform the development of panels that capture immune dysregulation at multiple levels, offering insights that can be translated into liver-specific contexts. By integrating these markers, CLRD moves toward a more holistic representation of immune activity rather than relying solely on static antibody titers.
Further strengthening this approach is CLRD’s work on pattern-recognition and cytokine signaling pathways. Studies have delineated the role of integrated stress response mechanisms in controlling IL-1β production and macrophage activation, processes that are central to inflammatory cascades in autoimmune diseases. Additionally, genetic investigations into mannose-binding lectin (MBL2) variants in inflammatory bowel disease provide a template for exploring similar genetic influences in autoimmune liver conditions. These mechanistic insights not only deepen our understanding of disease biology but also enable refined risk stratification, helping clinicians predict which patients may experience aggressive disease courses.
Together, these research-driven capabilities allow CLRD to configure an advanced immunology panel tailored for autoimmune liver diseases. This panel combines autoreactive antibody phenotyping with immune complex quantification and incorporates markers from stress-response and cytokine pathways. The result is a dynamic tool that captures the true activity state of the disease, moving beyond traditional serology to deliver actionable insights for diagnosis, prognosis, and therapeutic decision-making.
2) Exploring genetic and environmental triggers
Autoimmune liver diseases do not arise in isolation; they are the result of intricate interactions between genetic predispositions and environmental influences. Understanding these interactions is essential for predicting disease susceptibility and tailoring preventive strategies. CLRD has invested decades of research into decoding this complexity, building a robust foundation for gene environment mapping in autoimmune conditions.
On the genetic front, CLRD has led pioneering genotype–phenotype studies across a spectrum of inflammatory disorders. Investigations into DNA repair polymorphisms such as XRCC1 and APE1, along with variants in CD14 and macrophage migration inhibitory factor (MIF), have provided critical insights into pathways that regulate immune tolerance and inflammatory responses. Similarly, research on mannose-binding lectin (MBL2) genetics has highlighted innate immunity’s role in shaping disease risk. These findings collectively demonstrate CLRD’s capability to construct polygenic risk models and perform haplotype analyses for complex traits, a skill set now being applied to autoimmune liver diseases. By leveraging this expertise, CLRD can identify genetic markers that predispose individuals to autoimmune hepatitis or primary biliary cholangitis, enabling clinicians to stratify patients based on inherent risk.
Environmental factors add another layer of complexity to disease initiation and progression. CLRD’s extensive work on pathogen–host interactions offers a unique vantage point for exploring these influences. Studies on hepatitis B and C viruses, coupled with detailed genotyping of Helicobacter pylori and sero-epidemiological surveys, have illuminated how infections and hygiene practices modulate immune responses. These investigations underscore the importance of microbial and viral co-factors in triggering or exacerbating autoimmunity. By integrating these insights, CLRD is positioned to examine the role of gut microbiota, latent viral infections, and lifestyle exposures in shaping the trajectory of autoimmune liver diseases.
The convergence of genetic and environmental research enables CLRD to develop comprehensive gene–environment interaction maps. These maps serve as powerful tools for exposure-linked risk stratification, allowing clinicians to identify individuals at heightened risk long before clinical symptoms emerge. Such predictive capability informs preventive counseling, guides monitoring frequency, and supports early therapeutic intervention. Ultimately, this integrative approach transforms the management of autoimmune liver diseases from reactive treatment to proactive risk mitigation.
3) Non‑invasive biomarkers for real‑time monitoring: circulating NETs & cell‑free DNA
Monitoring autoimmune liver diseases requires more than periodic biochemical tests and invasive biopsies. These traditional approaches often fail to capture the dynamic nature of immune-mediated injury, leaving clinicians without timely indicators of disease activity. CLRD has addressed this challenge by developing and validating non-invasive biomarkers that provide real-time insights into tissue damage and immune activation, transforming the way autoimmune hepatitis and primary biliary cholangitis are managed.
One of the most promising tools in this domain is the measurement of neutrophil extracellular traps (NETs). CLRD’s research has established robust methodologies for quantifying NETs, including markers such as peptidyl arginine deiminase type 4 (PAD4), in high-inflammation settings like sepsis. These protocols are now being adapted to autoimmune contexts, where neutrophil-driven injury plays a significant role in disease exacerbation. In primary biliary cholangitis, NETs serve as indicators of cholestatic flares and vascular stress, while in autoimmune hepatitis, they reflect ongoing hepatocellular inflammation. By integrating NET quantification into routine monitoring, clinicians gain a sensitive marker for detecting subclinical activity and predicting impending flares.
Complementing NETs is the measurement of circulating cell-free DNA (cfDNA), another area where CLRD has demonstrated leadership. The center has validated workflows for simultaneous quantification of mitochondrial and nuclear cfDNA, applying these techniques to real-time disease assessment in cerebrovascular conditions. This experience translates seamlessly to autoimmune liver diseases, where cfDNA kinetics provide a window into hepatocyte injury and immune-mediated tissue destruction. Tracking cfDNA levels over time enables clinicians to monitor flare dynamics, evaluate therapeutic response, and adjust immunosuppressive regimens proactively, reducing the risk of irreversible damage.
In addition to NETs and cfDNA, CLRD’s work on endothelial dysfunction biomarkers such as soluble thrombomodulin and endoglin adds another dimension to disease monitoring. These markers, originally studied in critical illness, are highly relevant to the cholestatic microenvironment and fibrotic progression seen in primary biliary cholangitis. Their inclusion in monitoring protocols allows for early detection of vascular involvement, a key driver of disease severity.
Together, these innovations create a visit-independent monitoring layer that complements traditional biochemistry and autoantibody titers. By incorporating NETs, cfDNA, and endothelial markers into longitudinal care, CLRD enables a proactive approach to autoimmune liver disease management. This strategy not only improves the precision of disease tracking but also supports personalized immunomodulation, ensuring that therapy is guided by real-time biological signals rather than delayed clinical manifestations.
4) Translational models & regenerative platforms that strengthen clinical insight
Understanding autoimmune liver diseases requires more than observational studies; it demands experimental systems that replicate the complexity of immune-mediated injury and repair. CLRD has built such systems through its pioneering work in hepatic stem cell biology and organ bioengineering, creating platforms that bridge fundamental research and clinical application.
At the core of this capability is CLRD’s program on hepatic stem and progenitor cells. The center has successfully isolated and characterized human hepatic progenitor cells, including Epcam⁺ stem cell populations, and advanced their use in therapeutic transplantation. These achievements have transformed CLRD’s laboratories into living models of liver regeneration, enabling researchers to study the intricate cycles of autoimmune injury and tissue repair. By observing how these cells respond to inflammatory signals and immunomodulatory interventions, CLRD generates insights that inform both biomarker discovery and therapeutic innovation.
Complementing cellular studies is the development of bioengineered humanized livers. CLRD has created three-dimensional liver constructs and neo-liver grafts that mimic the architecture and function of native tissue. These engineered systems provide controlled environments to interrogate immune–biliary interactions, fibrosis pathways, and drug responses specific to autoimmune hepatitis and primary biliary cholangitis phenotypes. Unlike conventional in vitro models, these platforms allow for precise manipulation of variables, accelerating the translation of mechanistic findings into clinically relevant strategies.
Further enhancing this translational pipeline is CLRD’s expertise in in vivo tracking and exosomal analytics. The center has demonstrated non-invasive tracking of transplanted hepatic progenitors, ensuring real-time visibility into cell survival and integration. In parallel, research on cell-free exosome outputs from bioengineered livers offers a novel approach to monitoring immune states and repair signals during disease progression. These vesicles, rich in molecular cargo, serve as biomarkers and potential therapeutic agents, opening new avenues for personalized medicine.
Together, these regenerative and bioengineering platforms provide mechanistic clarity and enable rapid bench-to-bedside iteration. They support a continuum of innovation from identifying biomarkers that predict disease activity to testing immunomodulatory therapies and delivering regenerative solutions for advanced disease stages. For patients with autoimmune hepatitis and primary biliary cholangitis, this integrated approach promises not only better diagnostics and monitoring but also the possibility of restoring liver function through cutting-edge cellular therapies.
How CLRD Operationalizes This Specialization: A Roadmap
CLRD’s approach to autoimmune liver diseases is not limited to theoretical research; it is a structured, operational framework that translates scientific insights into clinical practice. This roadmap integrates multiple layers of diagnostics, monitoring, and translational feedback to ensure precision care for patients with autoimmune hepatitis and primary biliary cholangitis.
The foundation of this strategy lies in advanced serology and immune panels. Traditional autoantibody profiling, which includes markers such as ANA, SMA, LKM-1 for autoimmune hepatitis and AMA for primary biliary cholangitis, is expanded through CLRD’s research-driven enhancements. These panels incorporate immune complex quantification and cytokine or stress-response pathway readouts, providing a multidimensional view of immune activity. By moving beyond static antibody titers, CLRD enables clinicians to stage disease activity with greater accuracy and detect subtle shifts in immune dynamics that might otherwise go unnoticed.
Building on this foundation is the genomic and exposure layer, which addresses the underlying risk architecture of autoimmune liver diseases. CLRD employs targeted genotyping to identify variants in innate immunity and DNA repair pathways, alongside markers such as CD14 and MBL2 that influence immune regulation. These genetic insights are coupled with exposure analytics derived from decades of research on microbial and viral co-factors, including hepatitis viruses and Helicobacter pylori. The result is an individualized risk map that accounts for both inherited susceptibility and environmental triggers, guiding preventive counseling and informing the intensity of monitoring protocols.
The third pillar of CLRD’s operational model is real-time monitoring, which transforms disease management from episodic evaluation to continuous oversight. Through rolling assessments of neutrophil extracellular traps and cell-free DNA, clinicians gain actionable insights into flare prediction, tissue damage quantification, and therapeutic response. These biomarkers, validated in high-inflammation and ischemic contexts, are seamlessly adapted to autoimmune liver diseases, offering a visit-independent layer of surveillance that complements biochemical tests and serology. This capability ensures that treatment decisions are guided by dynamic biological signals rather than delayed clinical manifestations.
Finally, translational feedback closes the loop between research and care. CLRD leverages its bioengineered liver models and hepatic progenitor cell platforms to test mechanistic hypotheses, validate emerging biomarkers, and optimize immunomodulatory or anti-fibrotic strategies. These systems replicate the complexity of autoimmune injury and repair, enabling rapid bench-to-bedside iteration and accelerating the development of regenerative interventions for advanced disease stages.
Together, these four components, enhanced serology, genomic and exposure analytics, real-time biomarker monitoring, and translational modeling form a cohesive precision pathway. This roadmap ensures that CLRD’s specialization in autoimmune liver diseases is not only scientifically robust but also clinically transformative, delivering personalized care that adapts to the evolving biology of each patient.
CLRD’s Precision Pathway for Autoimmune Liver Conditions
The management of autoimmune liver diseases demands a paradigm shift from conventional, episodic care to a model that is dynamic, predictive, and deeply personalized. CLRD has operationalized this vision through a precision pathway that integrates multi-omic immunology, advanced biomarker science, and regenerative medicine platforms into a cohesive framework. This approach begins with early detection of autoreactivity using enhanced serology and immune panels, progresses through risk mapping informed by genetic and environmental analytics, and extends to real-time monitoring powered by circulating biomarkers such as neutrophil extracellular traps and cell-free DNA. These innovations ensure that disease activity is tracked continuously rather than intermittently, enabling clinicians to anticipate flares and adjust therapy before irreversible damage occurs.
Beyond diagnostics and monitoring, CLRD’s translational models and bioengineered liver systems provide mechanistic clarity and accelerate therapeutic innovation. By simulating immune-mediated injury and repair in controlled environments, these platforms allow rapid validation of biomarkers and optimization of immunomodulatory strategies. They also open the door to regenerative interventions for advanced disease stages, offering hope where conventional therapies reach their limits.
This research-anchored specialization transforms autoimmune liver care from static snapshots to a living continuum of data-guided management. It empowers clinicians with actionable insights at every stage, diagnosis, risk assessment, monitoring, and therapy, while maintaining a clear trajectory toward personalized and regenerative solutions. With this precision pathway, CLRD sets a new benchmark for the care of autoimmune hepatitis and primary biliary cholangitis, ensuring that science and clinical practice converge to deliver outcomes that were once considered aspirational.
