Peptic Ulcer Disease & Duodenal Ulcer
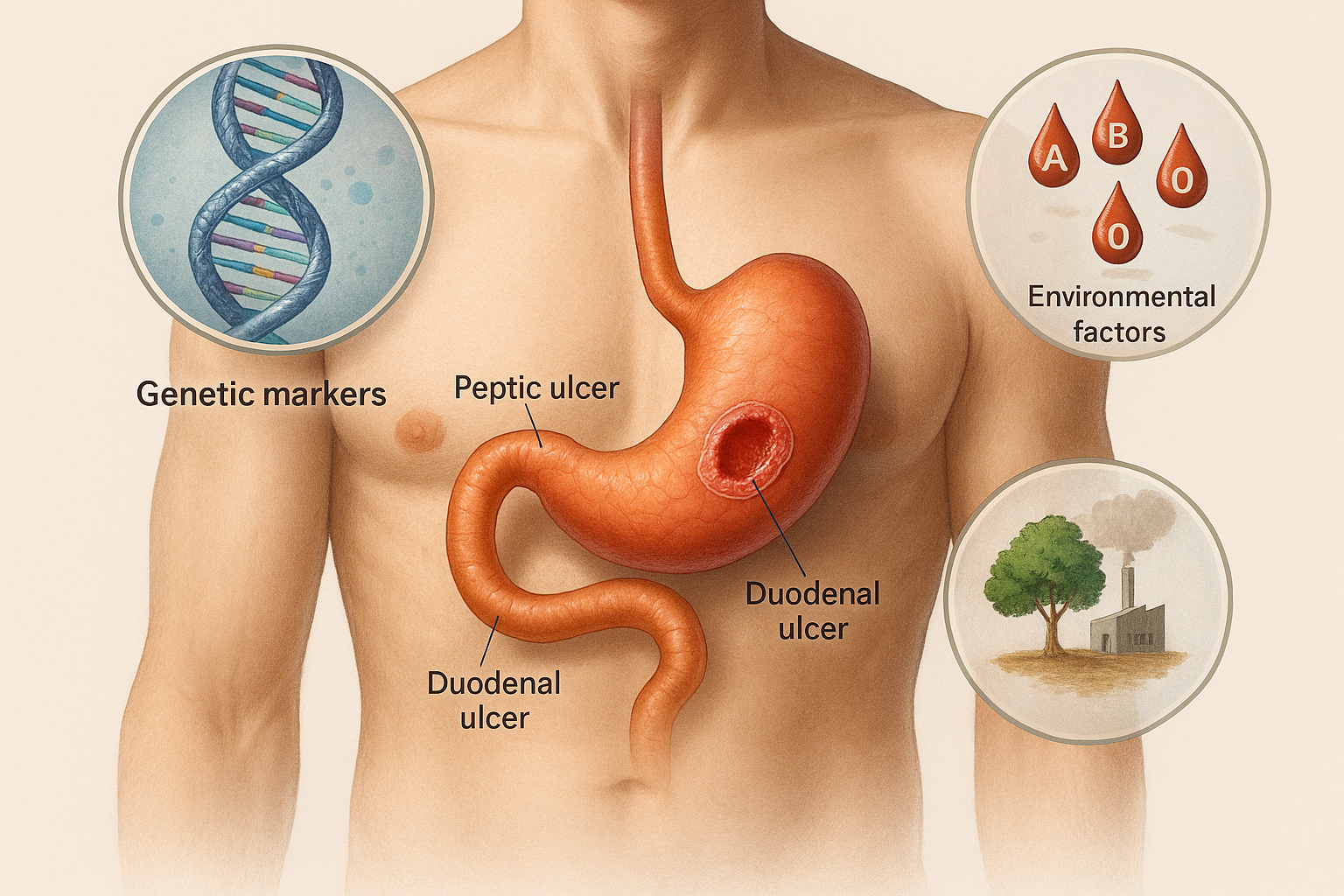
Peptic ulcer disease (PUD) and duodenal ulcer are among the most prevalent acid-peptic disorders affecting the upper gastrointestinal tract. These conditions are characterized by localized mucosal erosion that penetrates through the muscularis mucosa, typically occurring in the stomach (gastric ulcer) or the proximal duodenum (duodenal ulcer).
Pathophysiology
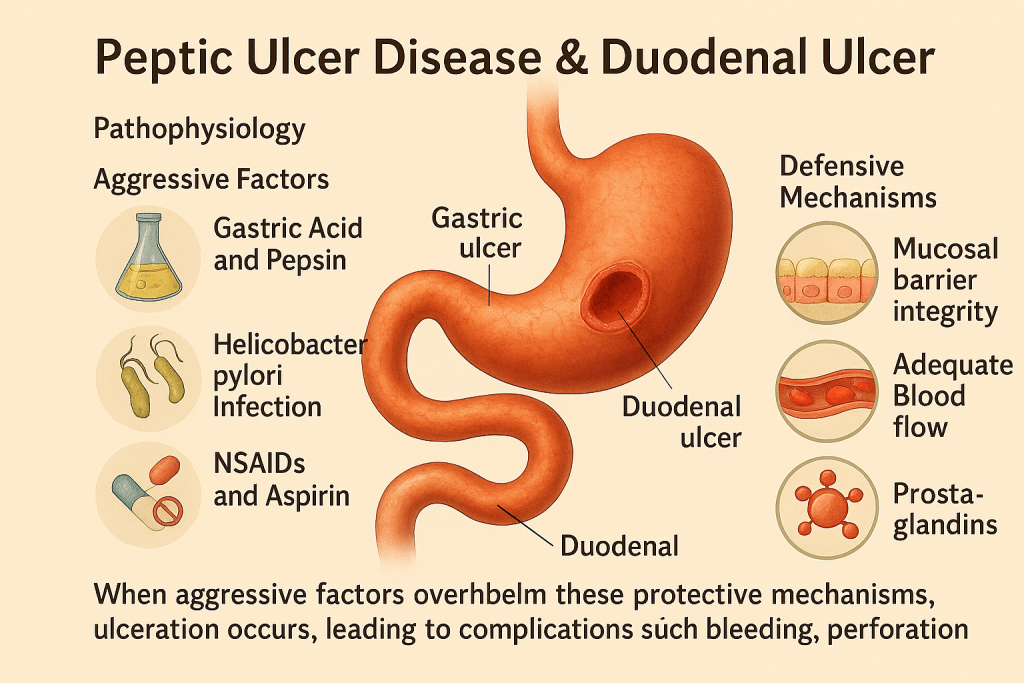
The development of these ulcers is primarily driven by an imbalance between:
Aggressive Factors
- Gastric Acid and Pepsin: Excessive secretion of hydrochloric acid and pepsin leads to mucosal injury.
- Helicobacter pylori Infection: A major etiological factor, H. pylori colonizes the gastric mucosa, producing cytotoxins (e.g., CagA, VacA) that disrupt epithelial integrity and stimulate acid secretion.
- NSAIDs and Aspirin: These drugs inhibit prostaglandin synthesis, reducing mucosal protection and increasing susceptibility to injury.
Defensive Mechanisms
- Mucosal Barrier Integrity: Composed of mucus, bicarbonate, and epithelial cell tight junctions that protect against acid damage.
- Adequate Blood Flow: Maintains tissue oxygenation and nutrient delivery for mucosal repair.
- Prostaglandins: Promote mucus and bicarbonate secretion and enhance mucosal defense.
When aggressive factors overwhelm these protective mechanisms, ulceration occurs, leading to complications such as bleeding, perforation, and gastric outlet obstruction.
Clinical Presentation
Symptoms:
- Epigastric pain (burning or gnawing), often related to meals.
- Duodenal ulcer pain typically improves after eating, whereas gastric ulcer pain may worsen post-meal.
Associated Features:
- Nausea, bloating, and early satiety.
- In severe cases: hematemesis (vomiting blood) or melena (black tarry stools).
Global Burden
Peptic ulcer disease affects millions worldwide, with significant morbidity and healthcare costs. In India, epidemiological studies, including those conducted by CLRD, highlight the interplay of genetic predisposition, dietary habits, and H. pylori prevalence in shaping disease patterns.
CLRD’s Research Contributions
The Centre for Liver and Digestive Research (CLRD) has played a pivotal role in advancing the understanding of PUD and duodenal ulcer through genetic, immunological, and environmental studies. Our landmark investigations have focused on:
Genetic Markers and Pepsinogen Polymorphisms
- CLRD identified pepsinogen gene variants as critical markers for ulcer susceptibility.
- Studies demonstrated that elevated serum pepsinogen levels correlate with increased gastric acid secretion, serving as a predictive biomarker for duodenal ulcer risk.
- Research published in Gut and Tropical Gastroenterology established pepsinogen genetics as a cornerstone for personalized risk assessment.
ABO Blood Group Correlations
- Our epidemiological studies revealed a strong association between blood group O and higher prevalence of duodenal ulcers.
- These findings provided insights into host genetic predisposition, influencing screening strategies in high-risk populations.
Environmental and Lifestyle Factors
- CLRD explored the role of dietary habits, stress, and occupational exposure in ulcer pathogenesis.
- Research on grape sprayers and pesticide exposure highlighted environmental triggers contributing to mucosal vulnerability.
Diagnostic Innovations
- Development of non-invasive biomarker assays for pepsinogen levels.
- Integration of genetic screening for pepsinogen polymorphisms and ABO typing in clinical workflows.
- Early adoption of endoscopic imaging protocols for precise ulcer localization and staging.
Therapeutic Insights
- CLRD’s clinical trials evaluated deglycyrrhizinated licorice (DGL) and other mucosal protective agents.
- Research on prostaglandin analogs and their role in ulcer healing informed pharmacological strategies.
- Studies on H. pylori eradication regimens optimized treatment outcomes for ulcer patients.
Impact and Global Relevance
- Our findings have influenced national guidelines for acid-peptic disorder management.
- CLRD’s work on genetic and environmental determinants is cited internationally, shaping preventive gastroenterology.
Future Directions
- Genomic risk profiling for ulcer prediction.
- Microbiome studies to understand host-pathogen interactions.
- Development of AI-driven diagnostic algorithms for early detection and personalized therapy.
Diagnostic Methods
Accurate diagnosis of peptic and duodenal ulcers is critical for effective management. CLRD has contributed significantly to the evolution of diagnostic strategies, combining traditional clinical approaches with innovative molecular techniques:
Endoscopic Examination
- Upper GI endoscopy remains the gold standard for visualizing ulcer sites, assessing severity, and ruling out malignancy.
- CLRD integrated high-resolution endoscopic imaging with biopsy protocols for histopathological confirmation.
Biomarker-Based Screening
- Serum Pepsinogen Assay: CLRD’s research established serum pepsinogen levels as a reliable biomarker for gastric acid secretion and ulcer risk.
- Urinary Pepsinogen Testing: Introduced as a non-invasive screening tool for early detection of ulcer diathesis.
Genetic Profiling
- Identification of pepsinogen gene polymorphisms and ABO blood group correlations for personalized risk assessment.
- Genetic screening incorporated into preventive programs for high-risk populations.
Helicobacter pylori Detection
- CLRD pioneered rapid diagnostic protocols, including:
- Urease-based tests (biopsy and breath tests).
- Salivary PCR assays for non-invasive detection.
- Multiplex PCR for genotyping virulent strains (cagA, vacA).
- CLRD pioneered rapid diagnostic protocols, including:
Imaging Techniques
- Contrast radiography for ulcer localization in resource-limited settings.
- Ultrasound adjuncts for complications like perforation or obstruction.
Advanced Molecular Diagnostics
- Development of ELISA and RPHA assays for H. pylori antigen detection.
- Genomic fingerprinting for strain-specific pathogenicity analysis, aiding prognosis and treatment planning.
